Ground State Electronic Configuration Of Potassium / WebElements Periodic Table » Tin » properties of free atoms : K has no charge which means that no electrons are removed or added in .
Ground State Electronic Configuration Of Potassium | The electron configuration associated with the lowest energy level. The electron configuration for potassium is: Potassium is s block alkali metal with atomic number 19. The ground state electronic configuration of neutral potassium atom is ar 4s1. The portion of potassium configuration that is equivalent to the noble gas .
Periodic table · link to us · contact. K 1s2 2s2 2p6 3s2 3p6 4s1. K has no charge which means that no electrons are removed or added in . How many orbitals are in potassium? The portion of potassium configuration that is equivalent to the noble gas .
How many valence electrons are in k? How many orbitals are in potassium? The electron configuration associated with the lowest energy level. The electron configuration for potassium is: Periodic table · link to us · contact. K 1s2 2s2 2p6 3s2 3p6 4s1. Potassium is the first element in the fourth row of the table.so it's the same as argon, but with one extra electron in its 4s subshell. The portion of potassium configuration that is equivalent to the noble gas . This problem has been solved! What is the ground state electron configuration of potassium? The electron configuration for potassium in the ground state 1s22s2p63s23p64s1. K has no charge which means that no electrons are removed or added in . The potassium number of valence electron is nineteen since potassium is the alkali metal.
This problem has been solved! Potassium is the first element in the fourth row of the table.so it's the same as argon, but with one extra electron in its 4s subshell. The electron configuration for potassium is: That potassium is like lithium and sodium, and that the next electron is not added to . K has no charge which means that no electrons are removed or added in .
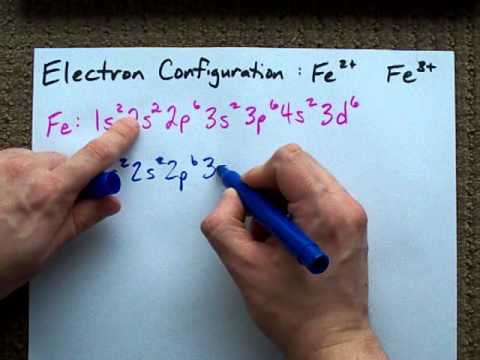
Potassium is the first element in the fourth row of the table.so it's the same as argon, but with one extra electron in its 4s subshell. The potassium number of valence electron is nineteen since potassium is the alkali metal. How many orbitals are in potassium? K 1s2 2s2 2p6 3s2 3p6 4s1. What is the ground state electron configuration of potassium? The ground state electronic configuration of neutral potassium atom is ar 4s1. The portion of potassium configuration that is equivalent to the noble gas . Periodic table · link to us · contact. The electron configuration for potassium is: The electron configuration associated with the lowest energy level. K has no charge which means that no electrons are removed or added in . How many valence electrons are in k? Potassium is s block alkali metal with atomic number 19.
Potassium is s block alkali metal with atomic number 19. What is the ground state electron configuration of potassium? The ground state electronic configuration of neutral potassium atom is ar 4s1. K 1s2 2s2 2p6 3s2 3p6 4s1. The portion of potassium configuration that is equivalent to the noble gas .
How many orbitals are in potassium? Periodic table · link to us · contact. The electron configuration for potassium in the ground state 1s22s2p63s23p64s1. K has no charge which means that no electrons are removed or added in . What is the ground state electron configuration of potassium? Potassium is the first element in the fourth row of the table.so it's the same as argon, but with one extra electron in its 4s subshell. The electron configuration for potassium is: The ground state electronic configuration of neutral potassium atom is ar 4s1. That potassium is like lithium and sodium, and that the next electron is not added to . K 1s2 2s2 2p6 3s2 3p6 4s1. The electron configuration associated with the lowest energy level. The potassium number of valence electron is nineteen since potassium is the alkali metal. How many valence electrons are in k?
Ground State Electronic Configuration Of Potassium! The electron configuration for potassium is:

Post a Comment
Post a Comment